Subscribe to Pittwire Today
Get the most interesting and important stories from the University of Pittsburgh.‘Shocking’ New Therapy May Be Key to Weakening Antibiotic Resistance
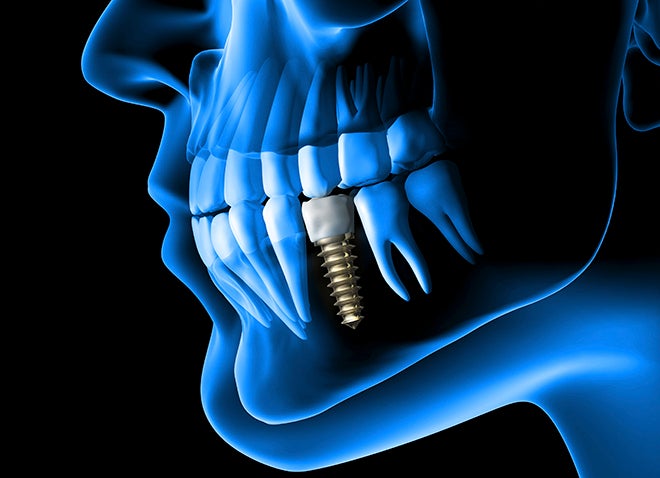
While titanium dental implants help improve lives each year, they can be costly. Replacing those implants can be costlier, and according to most recent numbers from a 2009 University of Southern California study, 5 to 10% of dental implants fail and must be removed within 10 to 15 years to prevent bacterial and fungal infection in patients, ranging from minor nuisance to fatal.
“We’re having a hard time with antibiotic resistance,” said Tagbo Niepa, assistant professor of chemical and petroleum engineering and bioengineering at the University of Pittsburgh Swanson School of Engineering. “This is a silent killer and we don’t seem to have the best technology to fight it.”
Niepa isn’t alone in that regard; the Centers for Disease Control and Prevention states at least 2 million people get an antibiotic-resistant infection annually, and at least 23,000 people die. Most antibiotics specifically work on active cells that are going to replicate, not dormant microbes—dormant microbes can cause infections to recur. Most antibiotics are also particularly weak against fungal infections.
Niepa recently had a study published in the journal American Chemical Society detailing a possible, “shocking” solution to this problem: electrotherapy.
This new research explains a process that would send a weak electrical current through the implant, such as the dental implant in the study. The current does not harm the patient or healthy tissue surrounding the implant, but is shown to weaken bacteria and other microorganisms to the point that antibiotics would eradicate them with ease.
The researchers focused their efforts on Candida albicans (C. albicans), one of the most common and harmful fungal infections associated with dental implants. They used the commonly used antibiotic fluconazole on solutions containing C. albicans cells or aggregates. Pre-electrotherapy, the antibiotic had a very low efficacy rate, about 3%. After introducing the electrical currents, the antibiotic’s efficacy had risen sharply to between 96 and 100%, meaning eradication of the microorganisms.
“This treatment would basically create ‘holes’ in the microbe’s cellular membrane, allowing medicine to enter and eliminate them,” Niepa said. “It also doesn’t pick and choose which microbes are targeted since they mainly damage the microbial cell’s membrane”—affecting both active and dormant microbes.
But while dental implants are one exciting application for this new technology, Niepa says it has other potential applications, such as joint implants and wound dressings.
“Most implants that are affected by microbial infections could be cleared using this technology,” said Niepa. “For soldiers, this could be useful in emergency battlefield situations where quick action is needed to prevent future infections.”
The research goes back to Niepa’s PhD candidacy work with eliminating harmful bacteria using similar methods. While no prototype device exists, Niepa will work with Pitt researchers specializing in dental medicine and surgery to test the therapy. Ideally, the final product will be used in clinical settings and not by the patients themselves, and will also be as non-invasive as possible.
“We’re excited to work with the Swanson School team on this new therapy,” said Charles Sfeir, associate dean for research in Pitt’s School of Dental Medicine. “Infections of dental implants (peri-implantitis) and other dental fixtures are a major problem for patients. Dr. Niepa’s research is innovative, so I’m glad he will be working with us. If successful, this could lead to better treatments for patients.”