Subscribe to Pittwire Today
Get the most interesting and important stories from the University of Pittsburgh.Computational Research Could Take the Guesswork Out of Creating New Metals
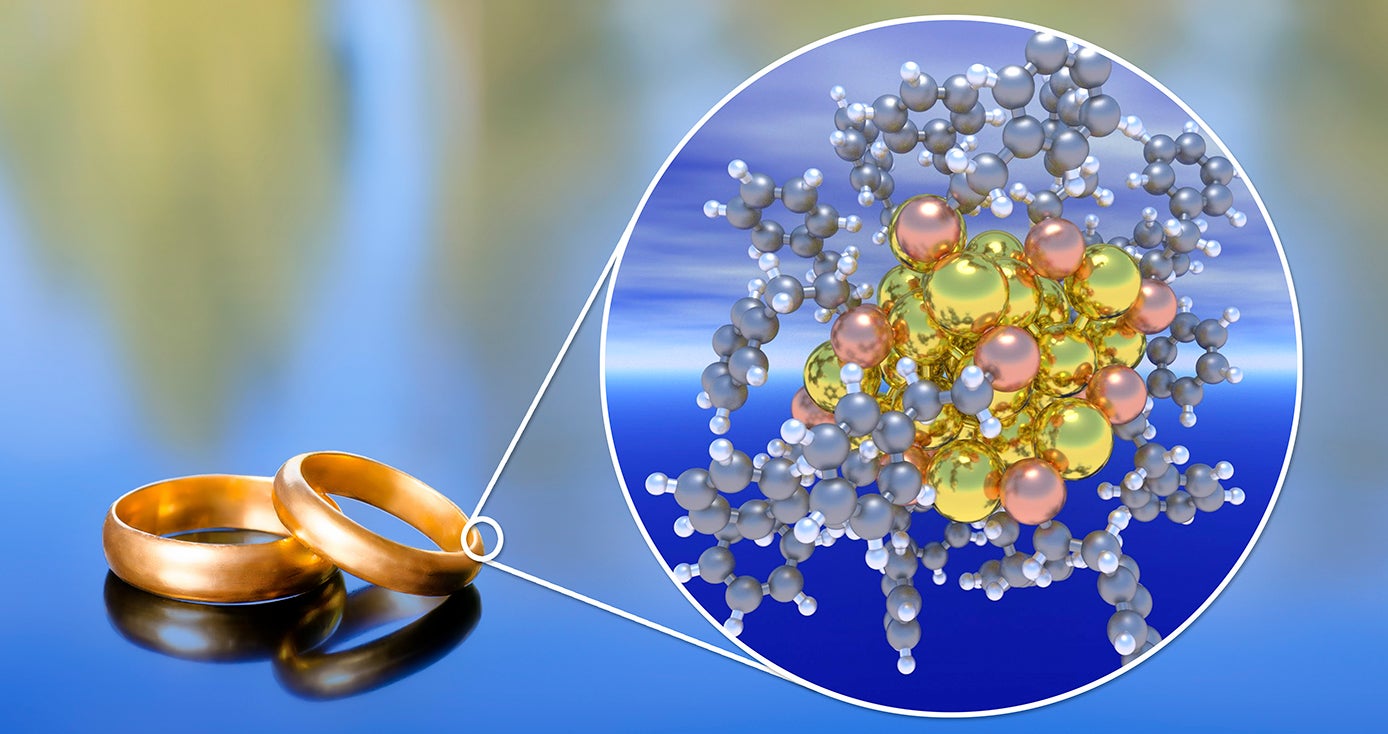
The steel beams that make up bridges and skyscrapers, the gold used for jewelry and the brass that forms musical instruments can be traced back to tiny building blocks invisible to the naked eye called metal nanoparticles — materials around 1,000 times smaller than the width of a human hair.
Scientists have been able to synthesize metal nanoparticles for years, but have not been able to figure out why they formed at specific sizes. This meant they had to rely on trial-and-error methods to make new kinds of metals needed for the aforementioned examples. In addition, no one is quite sure what makes these particles stable.
A new study in Nature Communications, co-authored by Pitt’s Giannis Mpourmpakis, an assistant professor of chemical and petroleum engineering at the Swanson School of Engineering, and PhD candidate Michael Taylor, offers a possible way to unravel these mysteries, with the help of computer simulations.
“In applying our new theory, we aim to accelerate discovery and application,” said Mpourmpakis. From molecular carriers for targeted drug delivery to systems for energy generation and storage to solar cells, this research could help.
At the crux of their work is something called a ligand — a molecule that binds to metal atoms, forming a metal core. That core is then stabilized by a shell. How the shell and the core interact, Mpourmpakis and Taylor found, determines the stability of the nanoparticle.
The energy in the metal core needs to interact with the shell at the same energy level to achieve stability, according to the research.
Previous theories describing why nanoclusters stabilized at specific sizes were based on the electron counting rules that would be familiar in any high school chemistry class. But some metal nanoclusters synthesized in the lab do not follow these rules.
Nanoparticle properties are related to their structures, Mpourmpakis said, and discovering novel, stable nanoparticle structures could potentially impact any field that uses these particles in developing new products or processes.
He also said that with this knowledge, researchers could create computer models of potential nanoparticles, eliminating that trial-and-error process during synthesis. This could help researchers create more efficient and sustainable production processes.
Mpourmpakis and Taylor have been working on this study since 2015. The duo said the next step focuses on follow-up studies aimed toward expanding the diversity of nanoparticles to which their stability theories can be applied.
The research, completed in Mpourmpakis’ Computer-Aided Nano and Energy Lab, is funded through a National Science Foundation CAREER award and bridges previous research focused on designing nanoparticles for catalytic applications.